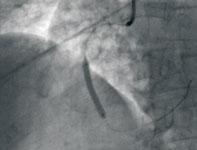
Photo B: The vessel was then predilated more completely using a 2.5 x 15 mm compliant balloon. Subsequently, a 3 x 28 mm bare metal stent (shown here).
Coronary revascularization does not always lead to coronary reperfusion. When readily available, percutaneous coronary intervention (PCI) using primary balloon angioplasty, with or without use of stenting, is the standard of care for ST-segment elevation myocardial infarction (STEMI). However, there is a group of patients who seem not to benefit fully from prompt restoration of antegrade flow, as they fail to show resolution of the indirect signs of ischemia such as electrocardiographic (ECG) changes and improvements in perfusion abnormalities.[1,2] These patients also present an angiographic phenomenon characterized by evidence of slow-flow in the affected vessel (Thrombolysis in Myocardial Infarction [TIMI] flow equal to or less than 2) and lack of contrast uptake “blush” by the subtended myocardium, leading to a potential dissociation between coronary revascularization and myocardial perfusion in STEMI.
This condition is referred to as “no-reflow phenomenon.” [1,2] According to Kloner et al,[3] no-reflow is defined as suboptimal myocardial reperfusion through a part of coronary circulation without angiographic evidence of mechanical vessel obstruction.
Mechanism and Pathogenesis of No-Reflow
This unique phenomenon is still poorly understood. However, theories have been generated trying to explain it. Originally, it was thought prolonged ischemia and extensive myocardial damage led to micro-vascular (capillary bed) damage, resulting in incomplete reperfusion.[3,4] More recently, other factors have been thought to play a role in the development of no-reflow, specifically distal embolization of the plaque/thrombus following balloon inflation. This theory was supported by the observation that patients with no-reflow have significantly greater amount of embolic material (platelet–fibrin complexes, cholesterol crystals and macrophages) trapped in the distal protection device when compared to patients with normal reflow.[5]
Other factors that contribute, at least partially, in the pathogenesis of no-reflow include systemic inflammatory response, platelet/endothelial activation, micro-vascular vasoconstriction, myocardial edema, oxygen-derived free radicals and calcium overload. Systemic activation of inflammatory cells might enhance no-reflow, as suggested by the observation that raised serum levels of C-reactive protein (CRP) are associated with impaired coronary micro-vascular response to both endothelium-dependent and endothelium-independent vasodilator stimuli[6,7] and with enhanced trans-cardiac neutrophil activation.[8] Platelets may be implicated in no-reflow through several mechanisms, including micro-vascular obstruction by platelet aggregates and release of platelet-derived vasoactive and chemotactic mediators.[9]
In summary, the cause of no-reflow can be classified into four main pathogenetic components: distal athero-thrombotic embolization, ischemia-related and/or reperfusion-related injury, as well as the susceptibility of coronary microcirculation to injury.[10] Although the exact mechanism of no-reflow remains unknown, it is most likely complex and multi-factorial.
Predictors of No-Reflow
Since the abovementioned theories were proposed, multiple possible predictors of no-reflow have been studied. Age, smoking, time-to-treatment interval, left ventricular ejection fraction (LVEF), previous myocardial infarction, Killip class, serum creatinine, CRP, B-type natriuretic peptide (BNP), baseline TIMI flow grade, and initial perfusion defect may all predict the development of no-reflow.[11, 12]
Because of the potential role of platelets in induction and perpetuation of no-reflow, mediators affecting platelet activation, such as thromboxane A2 (TXA2), might be involved in no-reflow. TXA2 is a key mediator of platelet activation and aggregation, and an important mediator of platelet-induced coronary artery constriction.[13, 14] Endothelin-1 (ET-1), a potent endothelium-derived vasoconstrictor peptide, might aggravate no-reflow by promoting reperfusion injury, triggering inflammatory response, and attenuating antioxidant defense.[15] In one study,[16] TXA2 plasma levels, ET-1 plasma levels and left anterior descending coronary artery (LAD) as the culprit vessel were significant predictors of angiographic no-reflow, whereas TXA2 and ET-1 plasma levels were the only independent predictors of lack of ST-segment resolution.
In another study,[17] patients with angiographically (TIMI flow equal to or less than 2), electrocardiographically (ST-resolution of less than 30 percent) and magnetic resonance imaging (MRI)-detected (presence of micro-vascular obstruction) no-reflow had significantly higher ET-1 level on admission. This was the only significant predictor of MRI-detected no-reflow together with left ventricular ejection fraction. An elevated ET-1 level was also a significant predictor of long-term mortality.
There are conflicting data about elevated serum levels of CRP (a marker of systemic inflammatory response) as a predictor of no-reflow. The association between elevated CRP level and no-reflow has not been consistently demonstrated.[11,12,18]
Potential Assessment Techniques
1) Index of Micro-Circulatory Resistance (IMR): Using a pressure/thermistor wire placed distally in the culprit vessel after PCI, IMR can be calculated as the product of distal coronary pressure and hyperemic transit time (derived by thermodilution during intracoronary papaverine or intravenous adenosine). In one study,[19] IMR correlated much better with peak creatine kinase (CK) and three-month echocardiographic wall motion score than TIMI frame count, myocardial blush grade, coronary flow reserve ratio or ST-segment resolution. An IMR less than 32 U was deemed reasonably good to identify patients whose wall motion score improved at three months. The authors concluded that IMR is an independent predictor of acute microvascular damage and late (three-month) LV functional recovery.
2) Myocardial Contrast Echo (MCE): The AMICI (Acute Myocardial Infarction Contrast Imaging) trial[20] compared MCE done within one day of PCI with TIMI flow grade, myocardial blush grade, peak CK, ST-segment resolution, and echocardiographic wall motion score. The end point was LV remodeling. The study showed only the endocardial length of the myocardial perfusion defect on MCE and a TIMI score less than 3 predicted LV remodeling.
Impact and Prognosis of No-Reflow
The development of no-reflow phenomenon is a poor prognosticator. It is associated with considerable reduction of the myocardial salvage by primary PCI in patients with STEMI. Because reduced myocardial salvage results in larger myocardial necrosis, no-reflow subsequently influences left ventricular function and mortality. In one study,[11] no-reflow after primary PCI was associated with reduced myocardial salvage, larger infarct size, and worse LV ejection fraction at six months.
One-year mortality was 16.7 percent in patients with no-reflow versus 5.5 percent in patients with normal flow. Six month follow-up angiography on patients with no-reflow showed that only 20 percent continued to have slow TIMI flow, with normalization of TIMI flow in the majority (80 percent) of patients. Patients with no-reflow after primary PCI who showed normalization of blood flow in the six-month angiography showed also a significantly better LV function than patients in whom suboptimal blood flow persisted six months after primary PCI. Recently, long-term prognostic data have also been published[21] and confirmed the persistent poor prognostic effect of no-reflow causing an increase in five-year mortality from 9.5 to 18.2 percent.
Prevention, Management
Although more research is much needed in this area and the precise pathophysiology is still to be determined, the simple application of the current standard-of-care can help prevent the development of no-reflow. These measures include reducing ischemic time (time-to-intervention) and administration of pre-procedural medications (aspirin, thienopyridine, heparin, beta-blocker, and high dose statin) in order to improve the micro-vascular integrity of the coronary bed.
The selective use of glycoprotein 2b-3a inhibitors and thrombectomy devices during intervention may be also appropriate in selected cases,[22] but lack of randomized controlled clinical trials remains a limitation to give specific guidelines. However, no-reflow can still occur even under the best provided care, which emphasizes the need for more specific mechanical and/or medical treatments. This is particularly true in the setting of an acute STEMI. Various intracoronary vasodilators have been shown to work with a good deal of success including nicardipine, nitroprusside and verapamil. The longer-acting drugs are somewhat limited by systemic hypotension and/or significant negative inotropy, both of which are of particular importance in the acutely ischemic heart. Two drugs in particular (nitroprusside and adenosine) have been studied as possible adjuncts to reperfusion therapy in multiple trials, including AMISTAD,[23, 24] ATTACC,[25] ADMIRE[26] and others.[27]
However, none of these studies has been performed in the setting of thrombus aspiration. The REOPEN-AMI (Randomized evaluation of intracoronary nitroprusside versus adenosine after thrombus aspiration during primary PCI for the prevention of no-reflow in acute myocardial infarction) study,[28] which is currently ongoing, should provide important data on the efficacy and safety of these vasodilators for prevention of no-reflow.
Conclusion
The no-reflow phenomenon remains a significant challenge for STEMI patients. It is associated with poor prognosis that is independent of and beyond that provided by other relevant clinical factors including the infarct size. Although we have a number of drugs and devices to treat the no-reflow phenomenon, there is an increasing need for continuation of research to better understand, prevent and treat this unique phenomenon in patients with STEMI undergoing primary PCI.
Editor’s note: Mark Michael, M.D., is chief fellow at the Krannert Institute of Cardiology, Indianapolis. Jeffrey A. Breall, M.D., Ph.D., is a professor of clinical medicine and director of the cardiac catheterization laboratories and interventional cardiology at the Krannert Institute of Cardiology. Breall also serves on the Editorial Advisory Board for Diagnostic & Interventional Cardiology.
References:
1. Hiroshi Ito. “No-reflow phenomenon and prognosis in patients with acute myocardial infarction.” Nature Clinical Practice of Cardiovascular Medicine, 2006; 3:499–506.
2. Arnoud W.J. van’t Hof, Aylee Liem, Harry Suryapranata, Jan C.A. Hoorntje, Menko-Jan de Boer, Felix Zijlstra, on behalf of the Zwolle Myocardial Infarction Study Group. “Angiographic assessment of myocardial perfusion in patients treated with primary angioplasty for acute myocardial infarction. Myocardial blush grade.” Circulation, 1998; 97:2302–2306.
3. Kloner RA, Ganote CE, Jennings RB. “The “no-reflow” phenomenon after temporary coronary occlusion in the dog.” Journal of Clinical Investigation, 1974; 54: 1496–1508.
4. Kloner RA, Rude RE, Carlson N, Maroko PR, DeBoer LWV, Braunwald E. “Ultrastuctural evidence of microvascular damage and myocardial cell injury after coronary artery occlusion: which comes first?” Circulation 1980;62:945–952.
5. Kotani J, Nanto S, Mintz GS, Kitakaze M, Ohara T, Morozumi T, Nagata S, Hori M. “Plaque gruel of atheromatous coronary lesion may contribute to the no-reflow phenomenon in patients with acute coronary syndrome.” Circulation, 2002; 106:1672–1677.
6. Tomai F, Ribichini F, Ghini AS, et al. “Elevated C-reactive protein levels and coronary microvascular dysfunction in patients with coronary artery disease.” European Heart Journal, 2005; 26:2099–105.
7. Schindler TH, Nitzsche EU, OlschewskiM, et al. “Chronic inflammation and impaired coronary vasoreactivity in patients with coronary risk factors.” Circulation, 2004; 110:1069–75.
8. Mazzone A, De Servi S, Ricevuti G, Mazzucchelli I. “Increased expression of neutrophil and monocyte adhesion molecules in unstable coronary artery disease.” Circulation, 1993; 88:800–3.
9. Heindl B, Zahler S,Welsch U, Becker BF. “Disparate effects of adhesion and degranulation of platelets on myocardial and coronary function in postischaemic hearts.” Cardiovascular Research, 1998; 38:383–394.
10. Niccoli G, Burzotta F, Galiuto L, et al. “Myocardial no-reflow in humans.” Journal of American College of Cardiology 2009;54:281-92.
11. Gjin Ndrepepa, Klaus Tiroch, Dritan Keta, Massimiliano Fusaro, et al. “Predictive factors and impact of no-reflow after PCI in patients with acute myocardial infarction.” Circulation: Cardiovascular Interventions, 2010; 3:27-33.
12. Young-Hoon Jeong, Won-Jang Kim, Duk-Woo Park, Seung-Jung Park, et al. “Serum B-type natriuretic peptide on admission can predict the ‘no-reflow’ phenomenon after primary drug-eluting stent implantation for ST-segment elevation myocardial infarction.” International Journal of Cardiology, 2010; 141:175–181.
13. Patrono C, Garc?´a Rodr?´guez LA, Landolfi R, Baigent C. “Low-dose aspirin for the prevention of atherothrombosis.” New England Journal of Medicine, 2005; 353:2373–2383.
14. FitzGerald GA. “Mechanisms of platelet activation: thromboxane A2 as an amplifying signal for other agonists.” American Journal of Cardiology, 1991; 68:11B–15B.
15. Niccoli G, Lanza GA, Shaw S, et al. “Endothelin-1 and acute myocardial infarction: a no-reflow mediator after successful percutaneous myocardial revascularization.” European Heart Journal, 2006; 27:1793-8.
16. Niccoli G, Giubilato S, Russo E, Spaziani C, et al. “Plasma levels of thromboxane A2 on admission are associated with no-reflow after primary percutaneous coronary intervention.” European Heart Journal, 2008; 29:1843–1850
17. Ingo Eitel, Marek Nowak, Clemens Stehl, et al. “Endothelin-1 release in acute myocardial infarction as a predictor of long-term prognosis and no-reflow assessed by contrast-enhanced magnetic resonance imaging.” American Heart Journal, 2010; 159:882-90
18. Giampaolo Niccoli, Gaetano A. Lanza, Cristina Spaziani, et al. “Baseline systemic inflammatory status and no-reflow phenomenon after percutaneous coronary angioplasty for acute myocardial infarction.” International Journal of Cardiology, 2007; 117:306–311
19. Fearon WF, Shah M, Ng M, et al. “Predictive value of the index of microcirculatory resistance in patients with ST-segment elevation myocardial infarction.” Journal of American College of Cardiology, 2008; 51:560–5.
20. Galiuto L, Garramone B, Scara A, et al., on behalf of the AMICI Investigators. “The extent of microvascular damage at myocardial contrast echocardiography is superior to other known indexes of post-infarct reperfusion in predicting left ventricular remodeling: results of the AMICI study.” Journal of American College of Cardiology, 2008; 51:552–9.
21. Gjin Ndrepepa, Klaus Tiroch, Massimiliano Fusaro, et al. “5-Year Prognostic Value of No-Reflow Phenomenon After Percutaneous Coronary Intervention in Patients With Acute Myocardial Infarction.” Journal of American College of Cardiology, 2010; 55:2383–9
22. Vetrovec GW. “Improving reperfusion in patients with myocardial infarction.” New England Journal of Medicine, 2008; 358:634–637.
23. Mahaffey KW, Puma JA, Barbagelata NA, DiCarli MF, Leesar MA, Browne KF, et al. “Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction: results of a multicenter, randomized, placebo controlled trial: the Acute Myocardial Infarction STudy of Adenosine (AMISTAD) trial.” J Am Coll Cardiol, 1999; 34:1711–1720.
24. Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW, AMISTAD-II Investigators. “A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II).” J Am Coll Cardiol, 2005; 45: 1775–1780.
25. Quintana M, Hjemdahl P, Sollevi A, Kahan T, Edner M, Rehnqvist N, et al. “Left ventricular function and cardiovascular events following adjuvant therapy with adenosine in acute myocardial infarction treated with thrombolysis, results of the ATTenuation by Adenosine of Cardiac Complications (ATTACC) study.” Eur J Clin Pharmacol. 2003; 59:1–9.
26. Kopecky SL, Aviles RJ, Bell MR, Lobl JK, Tipping D, Frommell G, et al. “A randomized, double-blinded, placebo-controlled, dose-ranging study measuring the effect of an adenosine agonist on infarct size reduction in patients undergoing primary percutaneous transluminal coronary angioplasty: the ADMIRE study.” Am Heart J, 2003; 146:146–152.
27. Amit G, Cafri C, Yaroslavtsev S, Fuchs S, Paltiel O, Abu-Ful A, et al. “Intracoronary nitroprusside for the prevention of the no-reflow phenomenon after primary percutaneous coronary intervention in acute myocardial infarction. A randomized, double-blind, placebo-controlled clinical trial.” American Heart Journal, 2006; 152:887.e9–887.e14.
28. Giampaolo Niccoli, Domenico D’amaria, et al. “Randomized evaluation of intracoronary nitroprusside vs. adenosine after thrombus aspiration during primary percutaneous coronary intervention for the prevention of no-reflow in acute myocardial infarction: the REOPEN-AMI study protocol.” Journal of Cardiovascular Medicine, 2009; 10:585–592.








 November 14, 2025
November 14, 2025 









